Water. How boring. It’s everywhere. There are massive bodies of it taking up most of the surface area of our planet. It is a simple molecule of two parts hydrogen and one part oxygen. No fancy elements. Nothing to see here.
Hold on! Water is far more interesting and downright weird than its public reputation suggests. If the properties of water were slightly different there would be no life on Earth. No life, no civilization, no you, no me, and worst of all this blog would not exist.
Biostasis advocates tend to frown on water, at least when it comes to preserving human biological systems. It has an annoying tendency to freeze, causing dehydration of cells. This increases the concentration of solutes within the cells while ice crystals grow between cells. Hence the need for cryoprotection and ultimately vitrification. Of course, we also know that water is crucial to life. But why is it so central to life? And what makes it unique?
Aqua mythos
Even before we get to water’s physical and chemical properties, we should acknowledge its mythological significance both ancient and recent. It may explain some of the odd takes on water-related science. Water is one of the four classical “elements”, a conception that endured for many centuries. Water has been regarded as medicine, as purifier, and as savior.
Water plays an outsize role in creation myths. This infinite, primeval ocean forms a central part of creation myths around the world. In Hawaiian creation myth an important role is played by Kanaloa, god of the ocean. In Māori myth: Tangaroa emerges from primordial darkness, creating the sea and is the great atua of the sea, lakes, rivers, and creatures that live within them. Then there is the Babylonian Enuma Elish story in which Primordial salt water (Tiamat) mates with fresh water (Apsu) to give birth to the gods.
In ancient Egyptian mythology, Nu (or Num) (“Watery One”) is the primordial waters from which all creation emerges, beginning with the creator god Ra. The Aztecs had Cipactli, a primeval sea monster, part crocodile, part fish, and part toad or frog. In Chinese mythology, Pangu emerges from a cosmic egg in primordial waters and separates heaven and earth. The aboriginal Australians envisioned ancestral spirits emerging from and shaping the landscape through water. And, of course, in the Judeo-Christian tradition, the Spirit of God stands over the waters until He separates them to create the sky before gathering them into the seas. Later, we find the famous story of Noah and the flood that wiped the Earth clean to start anew.
If these creation myths interest you, check out Gaston Bachelard Water and Dreams: An Essay On the Imagination of Matter which explores the mythic and legendary resonance of water.
Given the contemporary view that life originated in the oceans, it is curious that so many ancient myths reflect that narrative in their own way. Water expert Philip Ball, author of H2O: A Biography of Water, speculates that the cross-cultural Great Deluge myth may derive from the warming of the Earth 12,000 years ago. For the previous hundred thousand years, life had suffered through an ice age. So much water was confined in gigantic ice sheets that sea level was 390 feet lower than today. The rise in sea level took a thousand years – rather than the 40 days and nights of the Noah story – but the change would be noticeable in a lifetime and may have been preserved in oral history.
Water and pathological science
Philip Ball suggests that water’s mythical resonance may have driven the outbreaks of pathological science characterizing some supposedly scientific theories surrounding water. American scientist Irving Langmuir coined the phrase “pathological science” to describe both polywater and the “memory” of water.
Polywater was a hypothesized polymerized form of water first described by Soviet physicist Nikolai Fedyakin in 1961. Fedyakin measured the properties of water forced through narrow quartz capillary tubes. This polywater had the consistency of syrup or soft wax. In addition to its higher viscosity, this apparently new form of water had a higher boiling point, and lower freezing point than ordinary water. By the later 1960s, the popular press sparked fears of a “polywater gap” between the United States and the Soviet Union mirroring the “missile gap.”
Some suggested that polywater might be the most stable form of water at everyday temperatures and pressures. This fed a fear that a small amount of polywater dropped into the oceans might cause them to be converted into polywater with disastrous results. This fear remarkably mirrored Kurt Vonnegut’s 1963 novel, Cat’s Cradle, in which the fictitious ice-nine froze all the world’s seas. As we will see, there is an actual Ice IX but it has nothing to do with polywater. By the early 1970s, polywater was discredited, its supposedly unprecedented properties being due to impurities, most likely silicates leached from the glass.
In the 1980s, a new water-based bit of pathological science emerged. Jacques Benveniste and his collaborators experimented with high-dilution biological solutions and created the notion of the ‘memory of water’ in which water could supposedly become imprinted with biomolecular information. Benveniste claimed to find biological activity in solutions of antibodies so diluted that no dissolved molecules should remain. Strangely, this activity varied unpredictably depending on the degree of dilution. Also, the effect appeared only when the solution was shaken violently during dilution. The idea probably only seemed somewhat plausible to some because real uncertainties did (and do) exist about the structure of water. Inevitably, advocates of homeopathy jumped on the idea to justify their beliefs. The validity of these was tested and refuted, starting with a team led by John Maddox, editor of Nature.
Another weird science episode involving water was the claims about cold fusion. Chemists working in Utah claimed in 1989 that they had come up with a way of inducing nuclear fusion using nothing more than a beaker of heavy water and two palladium electrodes. A number of attempts to replicate the results seem to be promising initially but, over time, cold fusion was ruled out, although related experiments in low energy nuclear reactions (LENR) continue. With hindsight, some scientists assert that cold fusion should have been rejected from the start. The popularity of the idea may reflect the pervasive myth of water as fuel in the form of ‘water engines’ – machines that extract energy from water.
The heavy water used in cold fusion experiments, however, is a real thing. It is deuterium oxide, (2H2O, D2O), a form of water in which hydrogen atoms are all deuterium instead of the more common hydrogen-1 isotope (1H or protium). Heavy water is 10.6% denser than regular water and has a higher melting point. Heavy water lacks the slightly blue color of normal water and can taste slightly different. Compared to regular water, it has different biological effects and can kill multicellular organisms at concentrations over 50%.
Real water is still weird
Polywater and water memory may be weird science but water is genuinely weird. It seems incredible that water, that most familiar of liquids is, in 2024, far from fully understood. Disagreements and controversies abound.
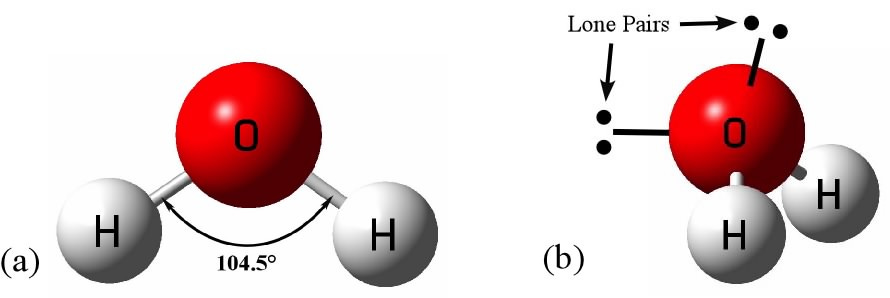
Water is the hydride of oxygen, each molecule an oxygen atom with two hydrogens attached. Even this agreed-upon statement raises questions when you compare it to the hydrides of other elements near oxygen in the periodic table. All the other hydrides – methane, ammonia, hydrogen sulfide, hydrogen chloride, and hydrogen fluoride are gases at room temperature and pressure (the last only in summer). Compared to the others, water is unusually resistant to evaporating. The molecules in other hydrides are not held together as strongly as a liquid.
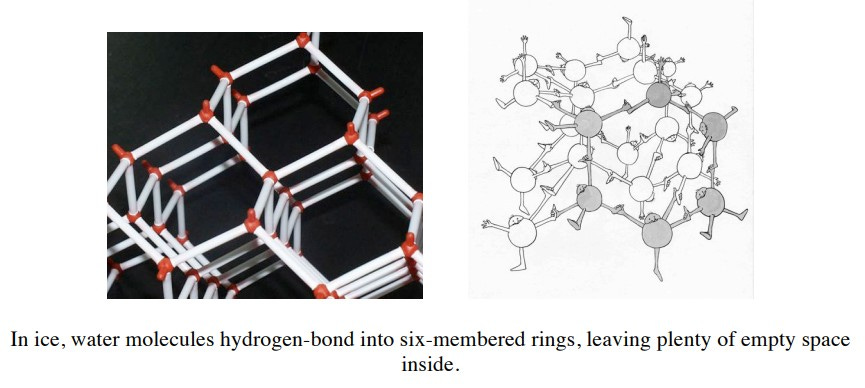
It is universally accepted that it is water’s fleeting hydrogen bonds that make it different from other liquids. These bonds restrict molecular reconfiguration but still continually break and form above water’s melting point. The hydrogen bonds can bend and deform to a degree but too much and the bonds will break. Much of water’s peculiarity stems from this balance between the open, orderly arrangement of the tetrahedral network and the random packing of the molecules. This interplay leads to unique thermal properties that cause water to behave differently from most other substances.
High specific heat capacity: Water can absorb or release a large amount of heat with minimal change in temperature. This enables water to stabilize climates by moderating temperature fluctuations in oceans, lakes, and the atmosphere. It also helps organisms maintain stable internal temperatures despite external temperature changes.
High heat of vaporization: Water requires a significant amount of energy to transition from liquid to vapor (~2260 J/g). This property provides an effective cooling mechanism, such as sweating or transpiration, by removing excess heat. It also plays a crucial role in the global water cycle, transferring heat from the surface to the atmosphere.
High heat of fusion: Water requires a substantial amount of energy to melt from solid (ice) to liquid (~334 J/g, or 80 calories/g). This prevents rapid freezing of water bodies, protecting aquatic life during cold conditions. It also acts as a buffer in seasonal transitions, slowing the freezing or thawing process.
Density anomaly (ice floats on water): We are all used to seeing ice float on water and don’t give it a second thought. But this is deeply unusual. Water contracts as it cools but, unlike anything else, once it reaches four degrees Celsius above the freezing point, it become less dense. Compare that to frozen carbon dioxide which sinks in liquid carbon dioxide or a lead ingot that sinks in a vat of molten lead. As a result, ice floats, forming an insulating layer on water bodies and protecting aquatic ecosystems from freezing solid and it maintains thermal stability in polar and cold regions.
What would happen if ice did not float? Life would be impossible. Lakes and oceans would freeze from the bottom up, making it impossible for seafloor or lake-bottom ecologies to exist except in equatorial zones. Once freezing got underway, bodies of water would remain frozen solid indefinitely since there would be no currents under the surface to promote melting in the spring. Glaciers have no current underneath and so remain for millennia on dry lands next to lakes. It is the tetrahedral structure that makes ice less dense than water.
High thermal conductivity: Water efficiently transfers heat compared to many liquids. This helps distribute heat evenly in oceans and lakes, promoting stable habitats, and it enhances heat transfer in biological systems, such as blood circulation.
High heat of vaporization (evaporative cooling): Evaporation removes heat from the surface, leading to cooling. Water regulates temperatures in organisms and the environment (for instance, through sweating or evaporation from water bodies).
High surface tension: Because of hydrogen bonding water has a higher surface tension than any other fluid except liquid selenium. This high surface tension draws it into rocks. When it freezes and expands, the rocks are broken apart. If water had a lower surface tension, soil formation would not occur. Water supports phenomena like capillary action and evaporation. If water had higher viscosity, circulatory systems could not evolve due to the lack of chemical processes that could power a heart to continually pump a more viscous fluid.
Wide temperature range: Water remains liquid between 0 °C and 100 °C (at 1 atm pressure). Water therefore supports diverse ecosystems by providing a stable liquid medium across a wide range of temperatures. It also makes water available for life and industrial processes.
Ice, ice, baby!
Not counting Vanilla Ice, there are at least 15 kinds of ice. These are helpfully named Ice I through Ice XV. They appear as ice is squeezed at different temperatures. Normal ice is Ice I and can be hexagonal (Ih) or cubic (Ic) in structure. Ice II has a rhombohedral structure and exists at high pressure and in a temperature range of -70 °C to -200 °C. Ice III is tetragonal with a dense, ordered structure making it harder than Ice I and requires a pressure of 200-300 MPa and a temperature range of -20 °C to -100 °C.
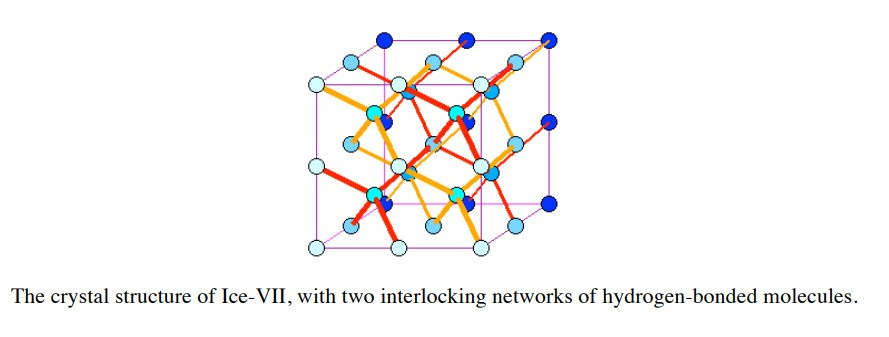
Ice IV is metastable in structure and rare, being made only in the lab at intermediate pressures. Ice V is monoclinic, meaning that the crystal is described by vectors of unequal lengths forming a parallelogram prism. Ice II to ICE XV exist only under extreme pressure and low temperatures. These are found in environments like the interiors of icy moons (e.g., Europa, Ganymede) and exoplanets. Ice VII and Ice X are considered to be “exotic ice” and have been found in diamonds from Earth’s mantle and could exist on super-Earths and water-rich exoplanets. Ice VII has two interlocking networks of hydrogen-bonded molecules. Ice XV comes into being only at -150 °C and a staggering pressure of 1-2 GPa (GigaPascals – normal atmosphere is 101 Pa).
Unlike the fictional ice-9, the real Ice IX is a polymorph of ice which melts at 45.8 °C (114.4 °F). Below that temperature, if Ice IX comes into contact with liquid water it acts as a seed crystal. The entire body of water will solidify and crystallizes as more ice-nine. If you somehow could ingest it, you would die nearly instantly. Fortunately, you need not worry about that because you would already be dead from the temperature and pressure.
Water under investigation
We have learned a great deal about water and have discovered curious forms of ice. But much remains to be understood and plenty of disagreement remains. There are still a few researchers who persist in looking into what they believe is water’s memory of molecular interactions or its supposed ability to form long-term structured clusters. That aside, here are some of the areas of research, experimental debates, and open questions.
How exactly do water molecules arrange themselves through hydrogen bonding? In the traditional view water forms a dynamic hydrogen-bonding network, where each molecule forms four hydrogen bonds in a tetrahedral structure. Recent studies using X-ray scattering suggest that water may not be purely tetrahedral and could feature transient, disordered clusters of bonded and non-bonded molecules. Some researchers argue for a dual structure with coexisting high-density (HDL) and low-density (LDL) liquid forms, though others maintain a more homogeneous hydrogen-bond network.
Does liquid water have two distinct phases at the molecular level? The two-liquid model suggests that water alternates between two distinct liquid states: one denser and more disordered, the other less dense and more structured. Some experiments using supercooled water and X-ray spectroscopy support this hypothesis, though the evidence remains contested. This could explain water’s density anomaly and other unusual properties.
What happens to water below its freezing point when it is supercooled? Water can remain liquid below 0°C under certain conditions. Its behavior in this state is highly debated, with some researchers suggesting it supports the two-liquid hypothesis. The exact molecular arrangement in glassy states is unclear.
Why does water expand when it freezes, unlike almost all other substances? Ice forms a crystalline structure with an open, hexagonal lattice due to hydrogen bonding, making ice less dense than liquid water. This leaves open the precise mechanism of how this lattice forms and why similar hydrogen-bonding liquids do not exhibit the same behavior.
Does quantum mechanics play a significant role in water’s molecular behavior? Some research suggests proton tunneling might occur in water’s hydrogen bonds, allowing protons to move between molecules in ways classical physics cannot explain.
Why does water behave so differently at interfaces and surfaces? Water exhibits unusual properties at interfaces, such as on hydrophobic surfaces (e.g., Teflon), where its hydrogen bonds reorganize. Researchers want to understand water’s interaction with surfaces because that is crucial for nanotechnology, materials science, and biological processes like protein folding.
Do clusters of water molecules persist longer than expected? Some researchers suggest that water molecules form temporary clusters or “flickering clusters” that persist briefly but influence water’s viscosity and diffusion rates. Others argue that these clusters are too transient and random to have significant effects on bulk water properties.
While much progress has been made, water remains scientifically mysterious, proving that even the simplest molecules can be complex and enigmatic. Even well into the 21st century we have much to learn about this most ubiquitous and familiar substance.